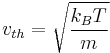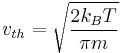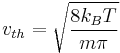Thermal velocity
The thermal velocity or thermal speed is a typical velocity of the thermal motion of particles which make up a gas, liquid, etc. Thus, indirectly, thermal velocity is a measure of temperature. Technically speaking it is a measure of the width of the peak in the Maxwell-Boltzmann particle velocity distribution. Note that in the strictest sense thermal velocity is not a velocity, since velocity usually describes a vector rather than simply a scalar speed.
Since the thermal velocity is only a "typical" velocity, a number of different definitions can be and are used.
Taking 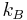 to be the Boltzmann constant,
to be the Boltzmann constant, 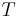 is the temperature, and
is the temperature, and 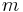 is the mass of a particle, then we can write the different thermal velocities:
is the mass of a particle, then we can write the different thermal velocities:
In one dimension
If 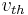 is defined as the root mean square of the velocity in any one dimension (i.e. any single direction), then
is defined as the root mean square of the velocity in any one dimension (i.e. any single direction), then
If 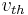 is defined as the mean of the magnitude of the velocity in any one dimension (i.e. any single direction), then
is defined as the mean of the magnitude of the velocity in any one dimension (i.e. any single direction), then
If 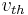 is defined as the
is defined as the 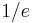 half-width of the thermal distribution or
half-width of the thermal distribution or
if 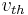 is defined such that a particle with this speed has an energy of
is defined such that a particle with this speed has an energy of 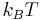 , then
, then
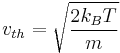 .
.
In three dimensions
If 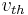 is defined as the root mean square of the total velocity (in three dimensions), then
is defined as the root mean square of the total velocity (in three dimensions), then
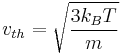 .
.
If 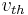 is defined as the mean of the magnitude of the velocity of the atoms or molecules, then
is defined as the mean of the magnitude of the velocity of the atoms or molecules, then